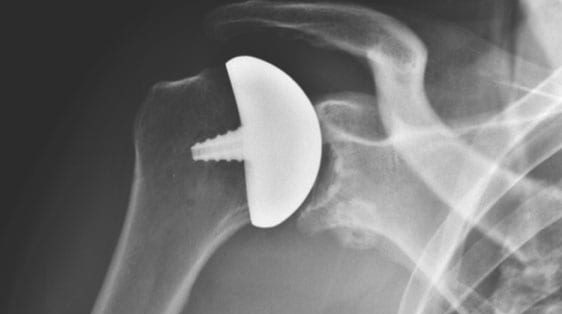
FRANKLIN, Mass. Oct. 11, 2018 /PRNewswire/ — Arthrosurface®, Inc. announced today the official launch of its latest product, the stemless OVOMotion™ Shoulder Arthroplasty System with Inlay Glenoid. FDA Cleared in April 2018, this stemless total shoulder system provides the surgeon with an approach that results in increased exposure to the glenoid, minimal bone removal, and is designed for patients with painful and/or severely disabled shoulder joints resulting from arthritis, traumatic events or AVN.
“This truly is a revolutionary stemless total shoulder, which combines the concept of anatomic restoration with an aspherical head design, and also allows improved joint access for ease of preparation of the glenoid,” said Dr. Anthony Miniaci, MD of the Cleveland Clinic. “This combination of an aspherical head and inlay glenoid has shown to be effective in not only active and younger patients but in all of those with glenohumeral arthritis even in the face of posterior subluxation and bone erosion.”
An earlier publication in the Journal of Shoulder and Elbow Surgery demonstrated how the inlay glenoid (socket) design was far superior to the only alternative with respect to the biomechanical stability. Arthrosurface has had long-standing success with the aspherical head design of the existing OVO® arthroplasty device and the stemless OVOMotion™arthroplasty system expands that technology by maintaining the same external geometry while adding a planar cut for enhanced access to the glenoid. Additionally, the proven fixation method and location are unchanged.
Michael Gombosh, MD from South Florida International Orthopaedics commented, “With the new system, the additional 12.5mm of bone removed from the humeral head has truly improved the technical portion of the procedure. This allows for easier visual and manual access of the glenoid. These steps can be the most challenging portion of the operation, and frequently the most time consuming. With improved visualization, you are able to obtain a more accurate assessment of the morphology and wear pattern within the glenoid, but also establish improved access for glenoid preparation and release of the posterior capsule.”
Steve Ek, CEO said, “The OVOMotion™ Shoulder Arthroplasty System builds upon the outstanding clinical results of the OVO System and greatly facilitates surgical access, speed and reproducibility. It also sets the stage for our future modular components.”
The OVOMotion™ Stemless Shoulder Arthroplasty System is now available for use in the United States.
About Arthrosurface
Arthrosurface, Inc. is a global orthopedic medical technology business providing a broad portfolio of essential products and instrumentation used to treat upper and lower extremity orthopedic conditions caused by trauma, injury and arthritic disease. The product offerings include joint preservation implants, instruments and orthobiologics. Founded in 2002, Arthrosurface markets and distributes its products in the US and around the world and has succeeded in helping patients return to activity for over 14 years. For more information, please visit our website at www.arthrosurface.com
SOURCE Arthrosurface, Inc.