Anika.
Restore Active Living.TM
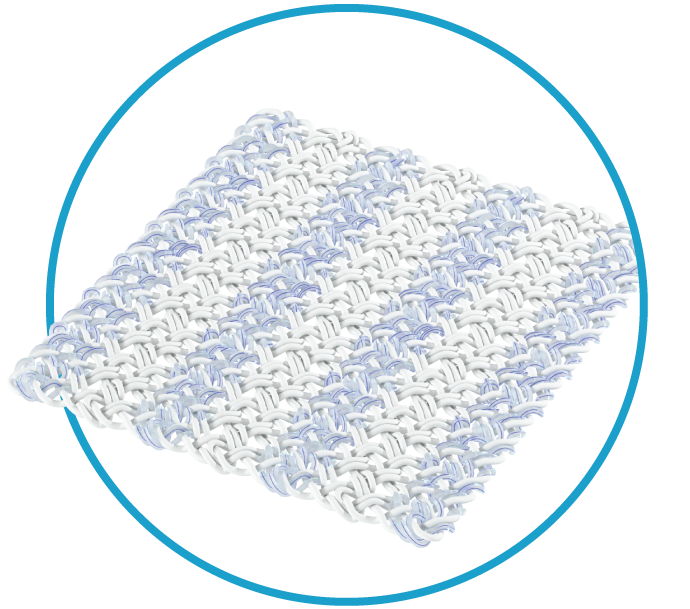
Introducing the Integrity™ Implant System, our newest regenerative solution. Integrity is a porous, knitted scaffold constructed of Anika’s Hyaff® material, a proven hyaluronic acid technology that supports tissue regeneration and resorbs over time, reinforced with non-absorbable PET (polyethylene terephthalate).
Anika.
Restore Active Living.®
Introducing the Integrity™ Implant System, our newest regenerative solution. Integrity is a porous, knitted scaffold constructed of Anika’s Hyaff® material, a proven hyaluronic acid technology that supports tissue regeneration and resorbs over time, reinforced with non-absorbable PET (polyethylene terephthalate).
Medical Professionals
We deliver a broad range of early intervention orthopedic solutions focused on joint preservation and restoration.
Patients
Learn more about our unique solutions for treating joint pain and arthritis, all designed and intended to restore active living.
Investors
We are committed to enhancing shareholder value through sustained profitable growth in early intervention orthopedics.
Our Vision
To be the global leader in joint preservation solutions that restore active living.
Our guiding principles are purpose driven and centered on customers and patients.
Join Anika
Everyone at Anika is passionate about helping people stay active and participate in the activities they love.
Let’s move forward together.
Integrity significantly enhances both the strength and biological support in rotator cuff repairs. Patient outcomes have been outstanding with postoperative imaging showing thickening of the rotator cuff repair. Patients are also consistently able to return to their desired activities in life without restrictions.”
Christopher E. Baker, MD, Florida Orthopaedic Institute
Learn More About Integrity