Tactoset Injectable Bone Substitute
Proprietary Hyaluronic Acid-Enhanced Formulation
Tactoset® provides a minimally invasive treatment option for bone marrow lesions or insufficiency fractures via percutaneous skeletal fixation.1 The product is used to improve the structural quality of the affected subchondral bone and promote local bone remodeling while preventing subchondral bone collapse and subsequent progression of osteoarthritis.
Tactoset is a synthetic, biocompatible, hyaluronic acid-enhanced, calcium phosphate bone graft substitute material intended for filling bone voids or defects of the skeletal system that are not intrinsic to the stability of bony structure. The device provides an injectable, self-setting, osteoconductive bone graft substitute that hardens post-deployment to reinforce weaknesses and mimic the properties of trabecular bone. Tactoset supports cell-mediated regeneration of new bone as it is resorbed and replaced by the growth of new bone during the healing process.2,3
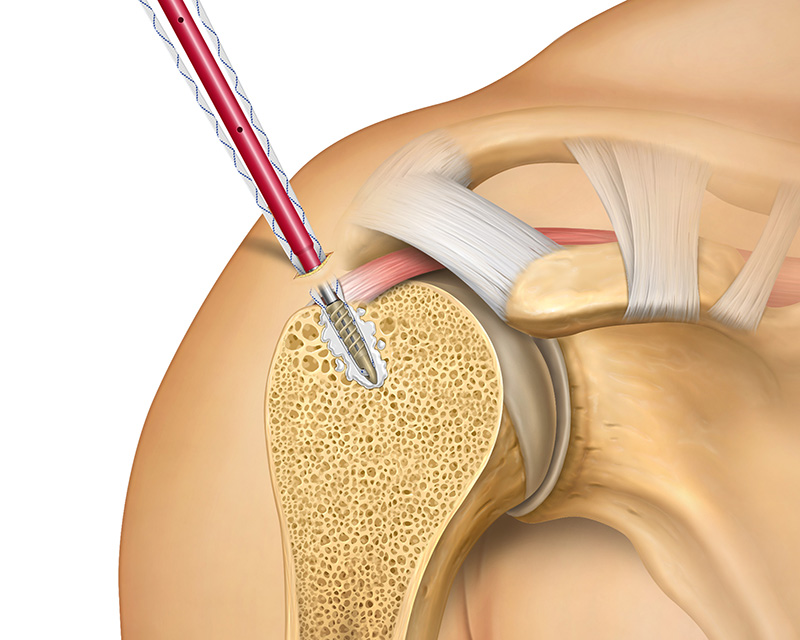
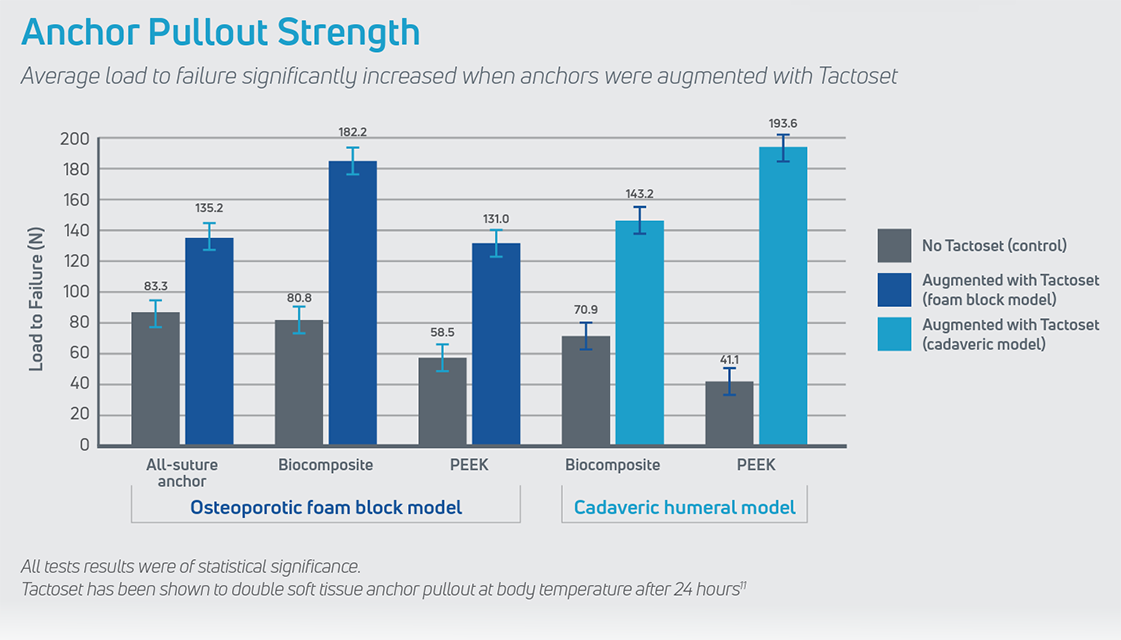
Tactoset is also indicated for the augmentation of hardware, such as soft tissue anchors, and can provide greater than twice the pullout strength of non-augmented anchors.3 Augmenting with Tactoset increases the strength of the fixation and the density of poor-quality bone caused by cysts or osteoarthritis, allowing for placement of anchors in the ideal repair location. Augmenting anchors with Tactoset was shown to significantly increase pullout strength and stiffness in osteoporotic bone models.4
Furthering the regenerative attributes of Tactoset, it is now indicated to be combined with autogenous bone marrow.2 Bone marrow aspirate (BMA) leverages a patients’ own biology and can help surgeons further address the spectrum of patient needs. Mesenchymal stem cells (MSCs), found within marrow, play a role in fracture repair by differentiating to become bone-forming osteoblasts and aiding in the remodeling processes.
Paired with Tactoset for its novel BMA indication, Anika offers the Marrow Cellution™ Bone Marrow Aspiration Needle, which extracts high-quality, high-cell-count marrow from multiple geographies while limiting peripheral blood dilution and eliminating the need for centrifugation.
Tactoset Injectable Bone Substitute, used to treat insufficiency fractures, improves the structural quality of subchondral bone, increases pullout strength of soft tissue anchors, and supports regeneration of new bone during the healing process.
Key Features & Benefits:
- Supports endogenous cell-mediated regeneration of new bone as material is resorbed2,3
- Hyaluronic acid-enhanced formulation
- Highly flowable and easily injectable during minimally invasive surgery1,3
- Interdigitates into trabecular bone architecture to fill closed bone voids1,3
- Indicated for augmentation of hardware, such as soft tissue anchors2
- Significantly increases pullout strength across various suture anchor types, improving the strength of the fixation4
- Unique cannula design allows surgeons to easily switch intraoperatively between side and end Tactoset delivery without having to remove and reinsert the outer cannula3
- Closed mixing system minimizes environmental exposure3
- Can be combined with bone marrow aspirate (BMA), leveraging a patient’s own biology2
- Can be performed as an outpatient procedure in an ambulatory surgical center (ASC)
Anika offers a comprehensive portfolio of soft tissue anchors in a broad range of size, material, and suture options to address rotator cuff repair and other surgical needs. View our Sports Medicine product offerings here.
Available in the U.S. Only
Anika products may not be available in all geographies. Product availability is subject to the regulatory clearances in individual markets. Please reach out to your local representative or Contact Us if you have questions about specific market approvals.
For complete product information, including indications, contraindications, warnings, and precautions, please refer to the Instructions for Use found here.
- Stark M, DeBernardis D, McDowell C, Ford E, McMillan S. Percutaneous Skeletal Fixation of Painful Subchondral Bone Marrow Edema Utilizing an Injectable, Synthetic, Biocompatible Hyaluronic Acid-Based Bone Graft Substitute. Arthroscopy Techniques 2020 Nov; Vol 9, No 11; pp e1645-e1650. doi:10.1016/j.eats.2020.07.005
- Tactoset Instructions for Use. Anika Therapeutics, Inc.; AML 500-335.
- Data on file, Anika Therapeutics, Inc.
- Diaz M, Munassi S, Teytelbaum D, Pipitone A, Baker C. An Injectable Calcium Phosphate Bone Graft Substitute Improves the Pullout Strength of Various Suture Anchor Designs in an Osteoporotic Bone Model. Arthroscopy, Sports Medicine, and Rehabilitation 2023 Jan 5; pp e1-e11. doi:10.1016/j.asmr.2023.01.010