Osteoarthritis Pain Management
Cingal: A One-Injection Combination of Hyaluronic Acid and Fast-Acting Steroid
Click here to visit the Cingal website:
Cingal websiteCingal® is the first and only approved combination viscosupplement formulated to provide the benefit of a cross-linked hyaluronic acid (HA) and a fast-acting steroid to effectively treat the symptoms associated with osteoarthritis (OA). It is a powerful, first-in-class treatment that combines the benefits of Anika’s proprietary HA formulation of Monovisc®, proven to deliver up to 6 months of relief of the symptoms of OA, with a well-established FDA-approved steroid, triamcinolone hexacetonide (TH), to treat inflammation and provide additional short-term pain relief.1
Cingal extends the benefits of Anika’s proprietary HA formulation to a broader range of patients across the spectrum of osteoarthritis pain.
Key Features & Benefits:
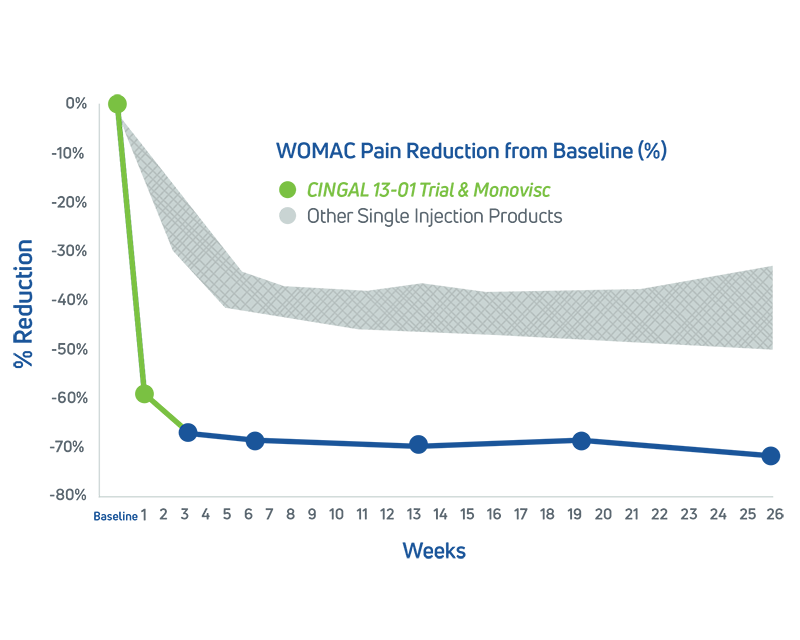
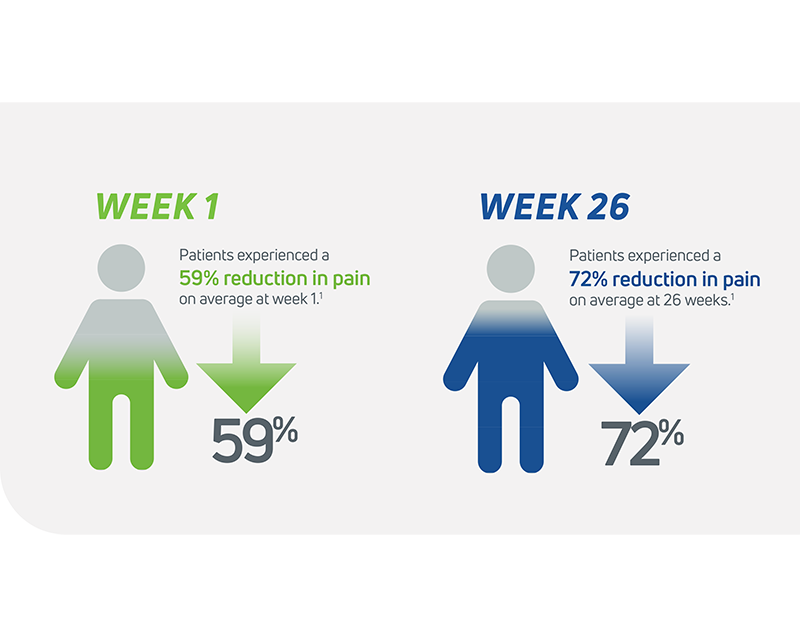
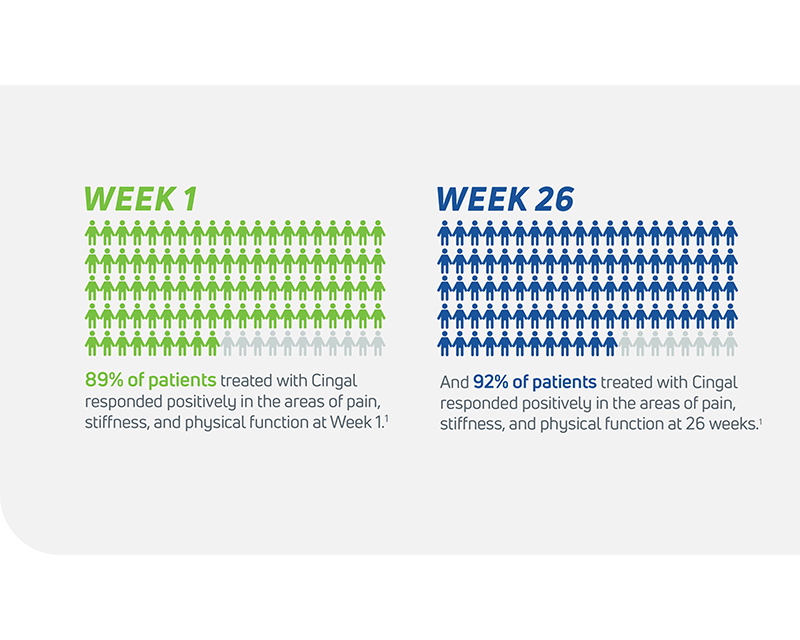
- In its 2014 pivotal clinical study, Cingal demonstrated an ability to provide rapid and long-lasting pain relief for those affected by knee OA.1
- Steroid provides fast, short-term pain relief within days of injection1
- HA viscosupplement provides long lasting pain relief, which may last through 6 months1
- Convenient, single-injection treatment
- Highly concentrated, non-animal-based HA
- Strong safety profile in both initial injection and repeat injections1,2
Cingal is not approved for use in the United States.
In the European Economic Area and other parts of the world, Cingal is indicated as a viscoelastic supplement or a replacement for synovial fluid in human knee joints. Cingal is well suited for rapid and long-term relief of the symptoms of human knee joint dysfunctions, such as osteoarthritis. The actions of Cingal are long-term relief of symptoms by lubrication and mechanical support supplemented by short-term pain relief provided by triamcinolone hexacetonide.
In Canada, Cingal is indicated for the treatment of pain in OA of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and to simple analgesics (e.g. acetaminophen). Cingal includes an ancillary steroid to provide additional short-term pain relief.
Anika products may not be available in all geographies. Product availability is subject to the regulatory clearances in individual markets. Please reach out to your local representative or Contact Us if you have questions about specific market approvals.
For complete product information, including indications, contraindications, warnings, and precautions, please refer to the Instructions for Use provided with the product or Contact Us for a copy.
- Hangody L, Szody R, Lukasik P, et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized; double-blind; place-controlled multicenter clinical trial [published online ahead of print May 1, 2017].
- Cartilage. doi: 10.1177/1947603517703732 CINGAL 13-02, an open-label, follow-on study to Cingal 13-01. Anika Therapeutics, Inc.: study sponsor, Dr. Laszlo Hangody: global principal investigator, SynteractHCR: CRO.