Osteoarthritis Pain Management
Click here to visit the Cingal website:
Are you suffering from knee pain due to osteoarthritis (OA) that is preventing you from doing the activities that you love? With the help of joint pain management products, you could be back to your active lifestyle in no time.
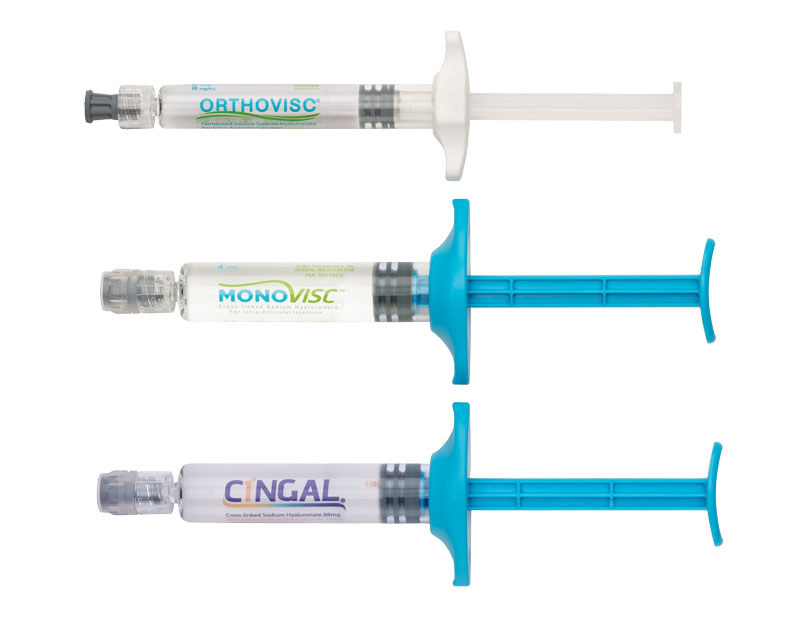
Orthovisc®, Monovisc® and Cingal®
OA is a degenerative joint disease and is the most common type of arthritis. OA occurs when the protective cartilage that cushions the ends of the bones breaks down over time. When your pain doesn’t respond to over-the-counter medications and exercise, your physician may recommend an injectable viscosupplement to reduce inflammation and boost joint lubrication.
Anika’s viscosupplement portfolio is comprised of three biocompatible hyaluronic acid-based therapies:
- Orthovisc reduces knee pain and improves functionality for up to 6 months with 3 to 4 injections
- Monovisc is a single injection treatment providing up to 6 months of knee pain relief
- Cingal combines the long-lasting pain relief of Monovisc with a well-established FDA-approved steroid to treat inflammation and provide additional short-term pain relief
Patient Benefits
- Ultra-pure, high molecular weight injectable hyaluronic acid
- Reduction in joint pain and improvement in functionality1-3
- Long-lasting results through 26 weeks1,3
- Helps patients return to an active lifestyle
If you are living with knee pain due to osteoarthritis, talk to your doctor about Anika’s joint pain solutions today.
Orthovisc and Monovisc are available in the United States and internationally. Cingal is not approved for use in the United States.
Orthovisc and Monovisc are available in the United States and internationally. Cingal is not approved for use in the United States.
Anika products may not be available in all geographies. Product availability is subject to the regulatory clearances in individual markets. Please reach out to your local representative or Contact Us if you have questions about specific market approvals.
For complete product information, including indications, contraindications, warnings, and precautions, please refer to the Instructions for Use provided with the product or Contact Us for a copy.
Resources
References
- Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intra-articular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin Orthop Relat Res 2001; (385):130 – 43.
- Neustadt D, Caldwell J, Bell M, Wade J, and Gimbel J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (ORTHOVISC) in osteoarthritis of the knee: A randomized, controlled, multicenter trial. J. Rheumatology 2005; (32): 1928-36.
- Hangody L, Szody R, Lukasik P, et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized; double-blind; place-controlled multicenter clinical trial [published online ahead of print May 1, 2017].