Integrity
Implant System
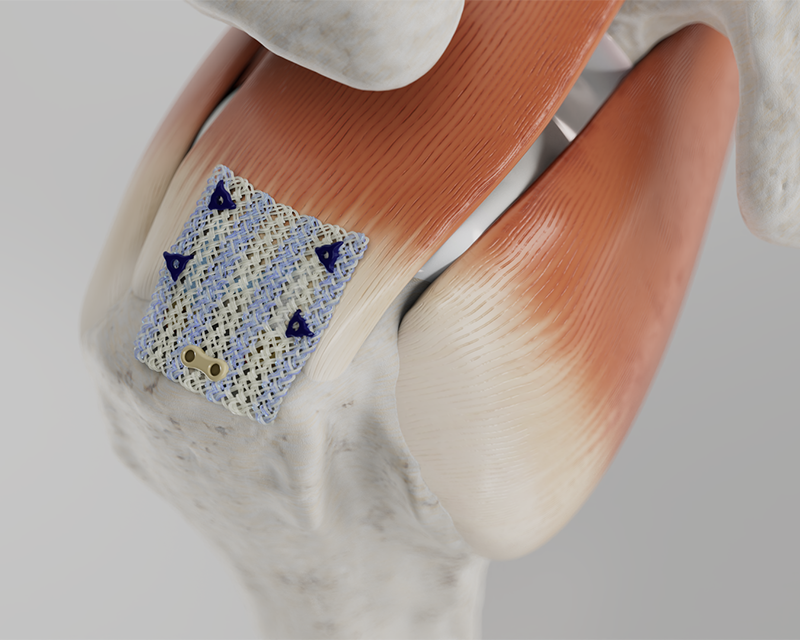
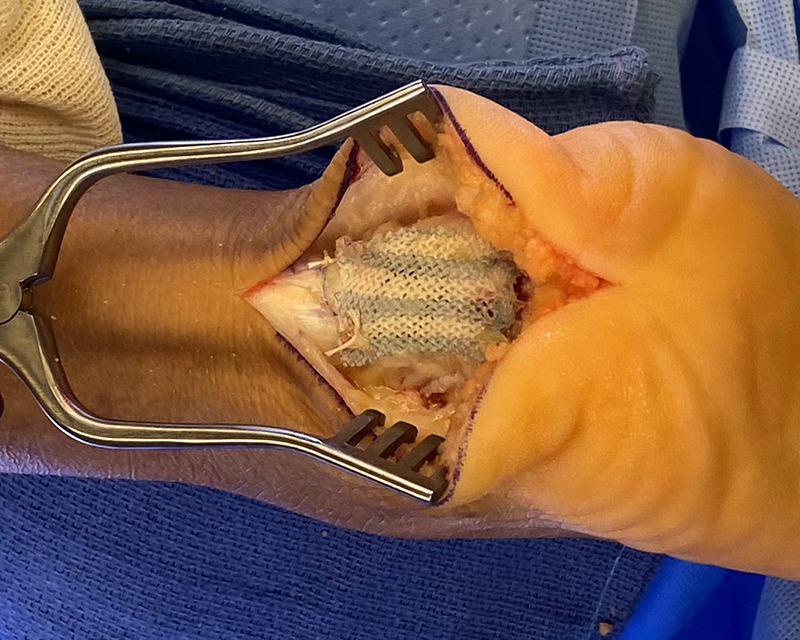
The Integrity™ Implant System is comprised of a hyaluronic acid-based implant fixated using PEEK bone staples, resorbable PLGA tissue tacks, or suture fixation, as desired, at the site of the rotator cuff augmentation.
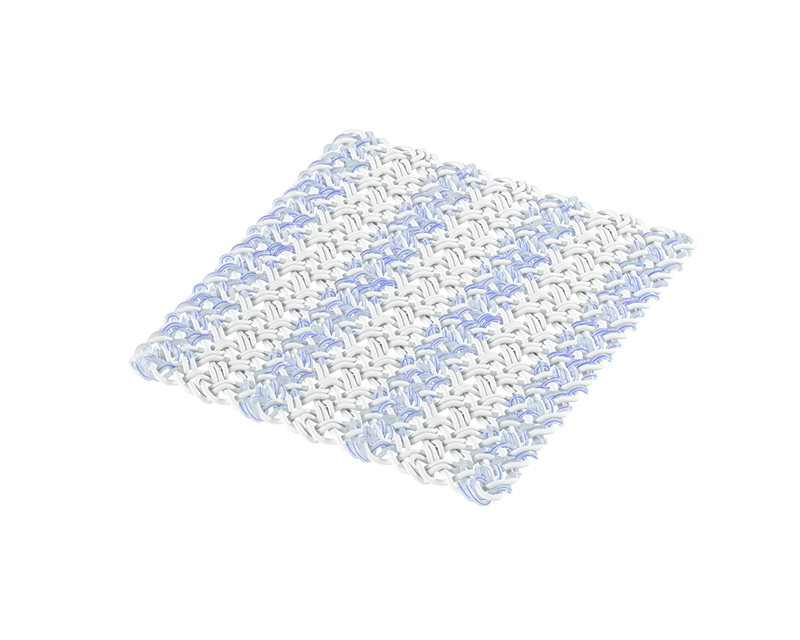
The Integrity Implant is a hyaluronic acid-based scaffold intended for rotator cuff repair, Achilles repair, and other tendon repair applications. It is a porous, knitted scaffold constructed of Hyaff® material, a proven hyaluronic acid technology that supports tissue regeneration and resorbs over time, reinforced with non-absorbable PET (polyethylene terephthalate).
Key Features & Benefits
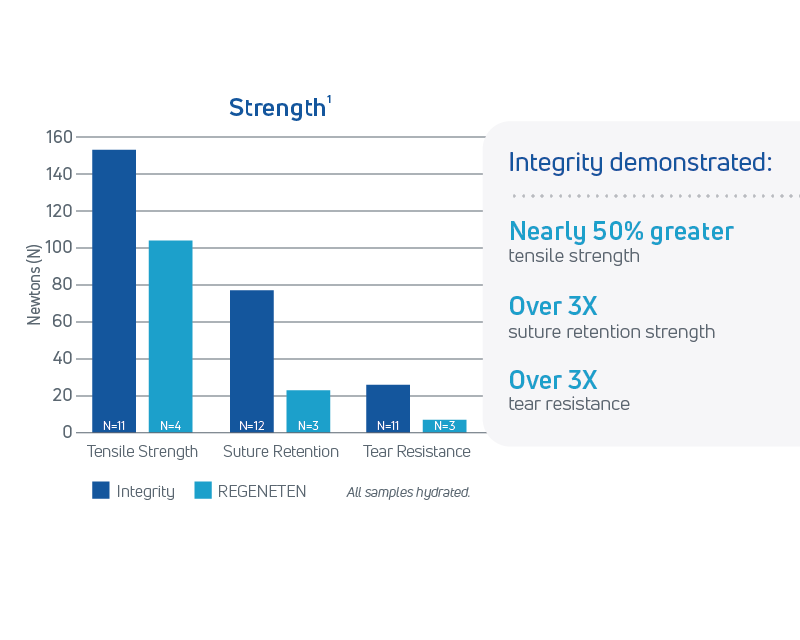
Reliable strength
- Integrity provides higher tensile strength, suture retention, and tear resistance in a thin knitted format1
- Inherently strong scaffold can be confidently manipulated arthroscopically and enables surgical versatility
Regenerative biology
- Hyaluronic acid-based scaffold supports regenerative healing through improved cell infiltration, tissue remodeling, and tendon thickening
- Porous, flexible construct knitted using Hyaff, Anika’s proven solid esterified hyaluronic acid technology that resorbs over time as tissue remodels
Streamlined technique for rotator cuff repair
- “Lateral first” fixation, unique instrumentation, and simplified surgical technique allow for precise implant placement
- Delivery instruments are single use and provided sterile for added efficiency
Foot & Ankle applications
- Achilles tendon
- Peroneal tendon
- Posterior tibialis tendon
Pre-clinical data
In a head-to-head pre-clinical study comparing Anika’s Integrity Implant and REGENETEN®, results showed:
- As early as 12 weeks post-implantation, fibroblast infiltration and regularly oriented new collagenous tissue formation had occurred within the Integrity repair, demonstrating greater regenerative capacity compared to REGENETEN1
- At 26 weeks, within the resorbing Integrity structure, new collagenous tissue infiltration forming a new network of tendon tissue had occurred, resulting in nearly 3 times greater thickness in the repaired tendon than REGENETEN1
- Increased tendon thickness is thought to improve the local biomechanical environment of the tear by reducing tendon strain, thus optimizing its healing potential2
The Integrity Implant System is available in the US.
The Integrity Implant System is available in select markets outside of the United States.
Anika products may not be available in all geographies. Product availability is subject to the regulatory clearances in individual markets. Please reach out to your local representative or Contact Us if you have questions about specific market approvals.
For complete product information, including indications, contraindications, warnings, and precautions, please refer to the Instructions for Use found here.
View More Products
- Data on file, Anika Therapeutics, Inc.
- Schlegal, T. F., M.D. (2017). Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: A prospective multicenter study. Journal of Shoulder and Elbow Surgery. https://doi.org/10.1016/j.jse.2017.08.023
REGENETEN is a registered trademark of Smith+Nephew.