Wrist Implants
WristMotion Total Wrist Arthroplasty System
The Next Generation of Total Wrist Arthroplasty
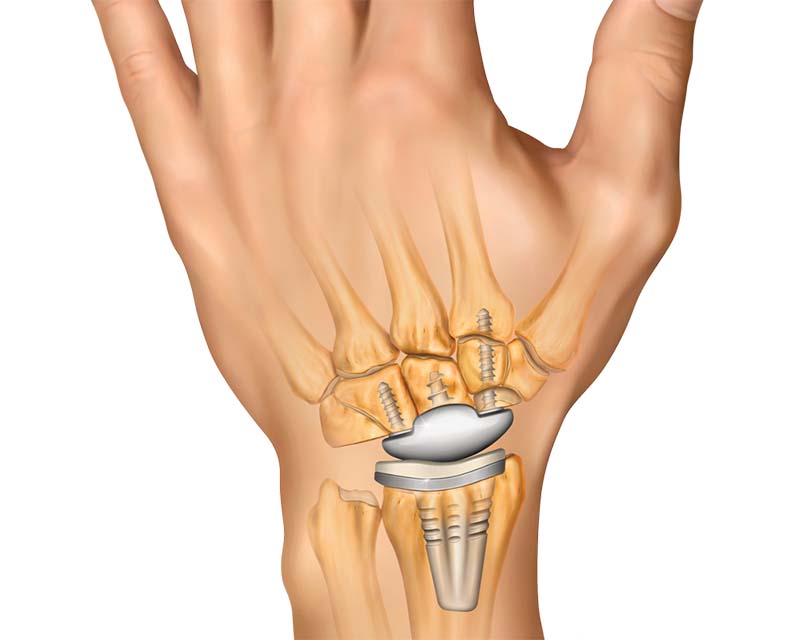
Designed to preserve motion and maximize carpal stability, the WristMotion Total Wrist Arthroplasty (TWA) System provides an advanced solution for wrist arthritis.
Key Features & Benefits:
- Secure carpal fixation with the proven center threaded taper post
- Improves range of motion and enables greater extension with the novel capitate cap
- Maintains the Dart Thrower’s Motion
- Preserves the center of rotation on the capitate by adjusting the height with radial polys
- Enhances implant stability with the precise, anatomically design implants that conform to the wrist anatomy
The WristMotion Total Wrist Arthroplasty System is intended to alleviate pain while restoring functionality and mobility for patients suffering from rheumatoid arthritis, osteoarthritis, or post-traumatic arthritis.
The WristMotion Total Wrist Arthroplasty System is available in the US only.
The WristMotion Total Wrist Arthroplasty System is not available outside the United States.
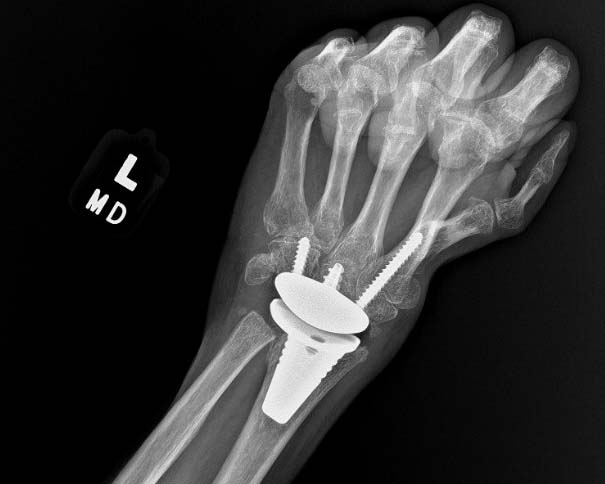
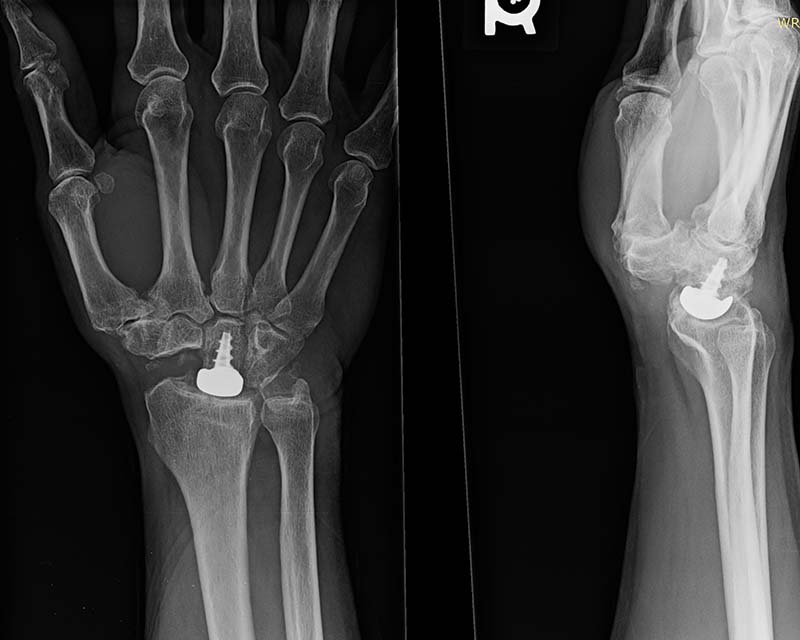
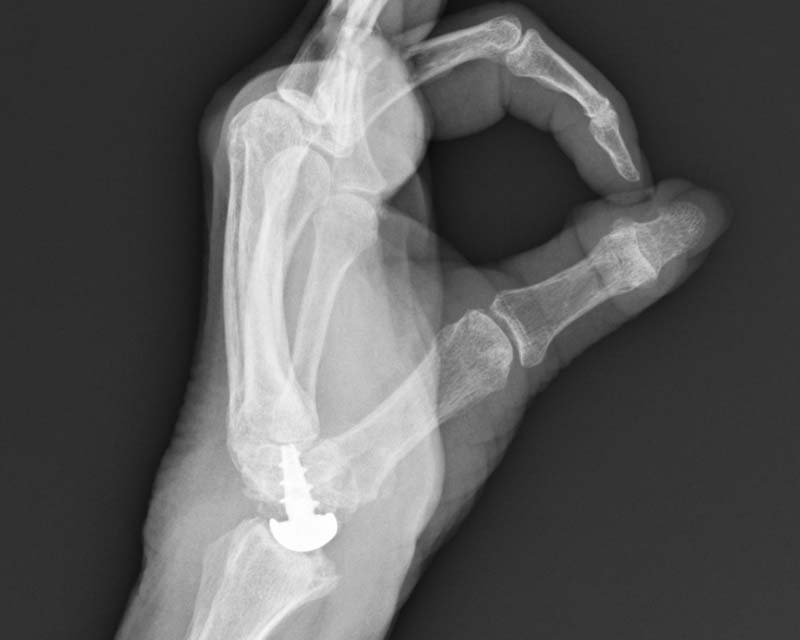
WristMotion Hemiarthroplasty System
Preserve and Prolong Motion
For the treatment of SNAC and SLAC wrists and irregularly shaped capitates, the WristMotion® Hemiarthroplasty System, paired with a Proximal Row Carpectomy (PRC), offers patients a solution that preserves and prolongs wrist motion.
Key Features & Benefits:
- Restores the capitate to the shape of the lunate for improved articulation
- Preserves range of motion and grip by maintaining joint height and native biomechanics
- Strong fixation with the center threaded taper post and morse taper connection
The WristMotion Hemiarthroplasty System is indicated for use as a partial replacement of wrist joint(s) disabled by pain, deformity and/or limited motion caused by: Non-inflammatory degenerative joint disease including osteoarthritis, traumatic arthritis and avascular necrosis; Rheumatoid arthritis; Revision where other devices or treatments have failed; Scapholunate Advanced Collapse (SLAC) and other functional deformities; Trauma, including fractures of the carpal bones.
The WristMotion Hemiarthroplasty System is available in the US.
The WristMotion Hemiarthroplasty System is available in select markets outside the United States.
Anika products may not be available in all geographies. Product availability is subject to the regulatory clearances in individual markets. Please reach out to your local representative or Contact Us if you have questions about specific market approvals.
For complete product information, including indications, contraindications, warnings, and precautions, please refer to the Instructions for Use found here.